Prenate Elite: Package Insert / Prescribing Info
Package insert / product label
Generic name: multivitamin/multimineral
Dosage form: tablet, coated
Drug classes: Iron products, Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Jan 11, 2024.
On This Page
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
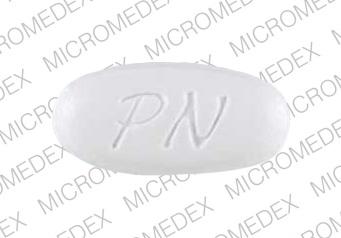
DESCRIPTION: PRENATE ELITE ® is a white, oval, oil- and water-soluble, multivitamin/multimineral, film-coated tablet debossed with “PN” on one side and blank on the other.
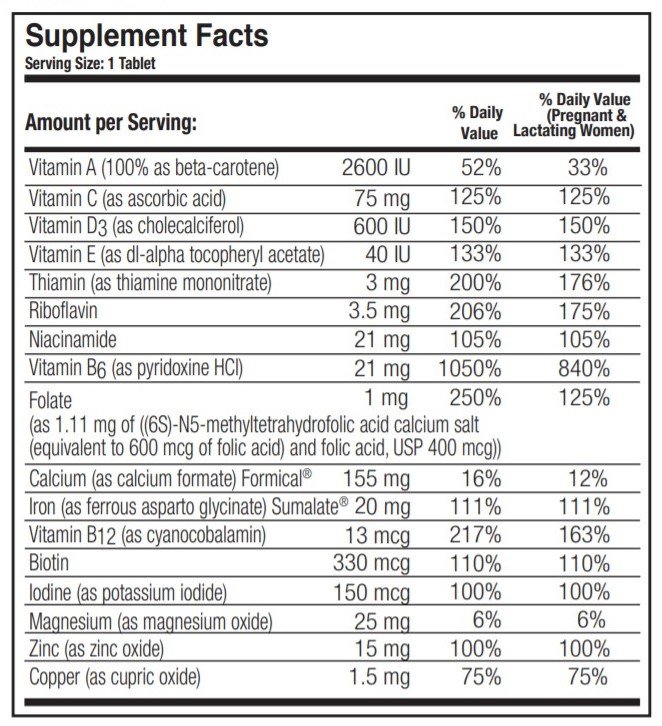
OTHER INGREDIENTS: Microcrystalline Cellulose, Silicon Dioxide, Croscarmellose Sodium, Stearic Acid, Magnesium Stearate, Film Coating (Hydroxypropyl Methylcellulose, Triacetin, Titanium Dioxide).
INDICATIONS: PRENATE ELITE ® is a multivitamin/multimineral fatty acid dietary supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers.
CONTRAINDICATIONS: PRENATE ELITE ® is contraindicated in patients with a known hypersensitivity to any of the ingredients.
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B 12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
DOSAGE AND ADMINISTRATION: Before, during and/or after pregnancy, one tablet daily or as directed by a physician.
HOW SUPPLIED: Bottles of 30 tablets (75854-314-30). The listed product number is not a National Drug Code. Instead, Avion Pharmaceuticals has assigned a product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature.]
MANUFACTURED FOR:
Avion Pharmaceuticals, LLC
Alpharetta, Georgia 30005
1-888-61-AVION
Rev. 0519-01
Formical ® is a registered trademark of Nephro-Tech 1, LLC, covered by one or more claims of U.S. Patent No. 6,528,542.
Sumalate ® is a registered trademark of Albion Laboratories, Inc., covered by one or more claims of U.S. Patent Nos. 5,516,925, 6,716,814, 8,007,846, and 8,425,956.
| PRENATE ELITE
.beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, 5-methyltetrahydrofolic acid, calcium formate, ferrous asparto glycinate, cyanocobalamin, biotin, potassium iodide, magnesium oxide, zinc oxide and cupric oxide tablet, coated |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Avion Pharmaceuticals, LLC (040348516) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avion Pharmaceuticals, LLC | 040348516 | manufacture(75854-314) | |
More about Prenate Elite (multivitamin, prenatal)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- Drug class: iron products
Professional resources
Other brands
Prenatal 19, Vitafol Ultra, Prenatal Plus Iron, PreNexa, ... +35 more